The mechanical properties of metal materials are the behaviors of metal materials under external loads or the combined effects of loads and environmental factors (temperature, medium and loading rate).
The mechanical properties of common metals are shown in the following table:
| Mechanical properties of metals | Mechanical property indexes of common metal |
| Strength | Yield strength, tensile strength, breaking strength |
| Plasticity | Elongation, reduction of area, strain hardening index |
| Resilience | Modulus of elasticity (stiffness), elastic limit, proportional limit |
| Hardness | Brinell hardness, Vickers hardness, Rockwell hardness |
| Toughness | Static toughness, impact toughness, fracture toughness |
| Fatigue | Fatigue strength, fatigue life, fatigue notch sensitivity |
| Stress corrosion | Critical stress field intensity factor and stress corrosion crack growth rate for stress corrosion cracking |
Stress-strain curve of low carbon steel unde uniaxial tensile loading
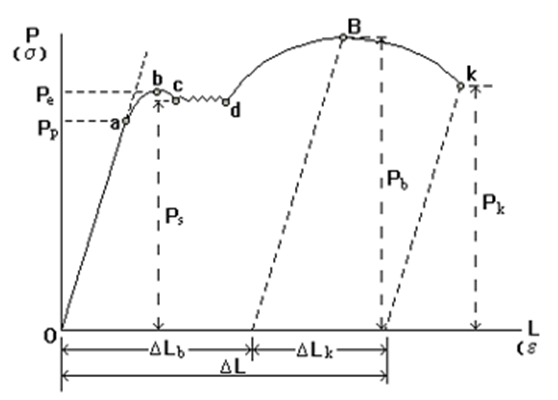
1. Section OA: elastic deformation
2. Section ab: elastic deformation + plastic deformation
3, BCD section: obvious plastic deformation, yield phenomenon, continuous elongation of the sample under the condition that the force is basically unchanged.
4. Curve of dB section: elastic deformation + uniform plastic deformation
5. Point B: necking occurs, the local section of the sample is significantly reduced, the bearing capacity of the sample is reduced, the tensile force reaches the maximum value, and the sample is about to break.
Strength Index
Strength is the ability of a material to resist plastic deformation and fracture.
1. Yield strength
σs = Fs/S0
Fs: tensile force (N) when the specimen yields; S0: Original cross-sectional area of the specimen (mm).
2. Tensile strength
The maximum tensile stress sustained by the specimen before fracture reflects the resistance to the maximum uniform deformation of the material.
σb = Fb/S0
σbis often used as a basis for the selection and design of brittle materials.
Plasticity index
Plasticity is the ability of a material to deform plastically without failure under static loading.
1. Elongation after fracture
The percentage of the elongation of the gauge length after the sample is broken to the original gauge length.
δ=(L1-L0)/L *100%
L0: gauge length; L1: gauge length of test piece after tensile fracture.
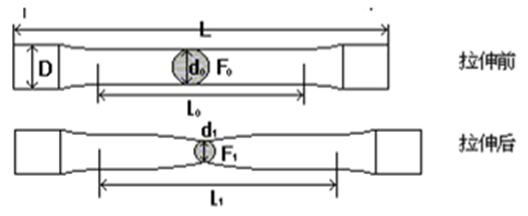
2. Reduction of area
The percentage of the maximum reduction in cross-sectional area of the specimen at the reduced item after tensile fracture to the original cross-sectional area.
Ψ=(A0-A1)/A0 *100%
A0: original cross-sectional area of the test piece; A1: cross-sectional area at the necking after fracture.
Elasticity index
Stiffness: The ability of a material to resist elastic deformation when subjected to a force.
E=σ/ε
σ: tensile stress; ε: Tensile strain
Alloying, heat treatment and cold plastic deformation have little effect on the mechanical properties which are not sensitive to the structure.
Important mechanical property indexes for mechanism and component selection:
► The crane beam shall have sufficient rigidity, otherwise it will cause vibration due to excessive deflection when lifting heavy objects.
► Machine and press spindles, beds and worktables have requirements for rigidity to ensure machining accuracy.
► Main components of internal combustion engine, centrifuge and compressor shall have sufficient rigidity to prevent vibration.
Hardness
The ability of a local surface of a material to resist plastic deformation and failure.
It is an index to measure the softness and hardness of materials, and its physical meaning is related to the test method.
Hardness test methods: Brinell hardness, Rockwell hardness, Vickers hardness, Shore hardness, Leeb hardness and Mohs hardness
(1) Brinell hardness
The average stress per unit area, i.e., the quotient of the test force p and the spherical surface area of the indentation.
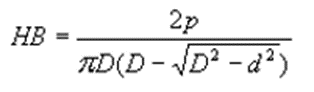
< 450HB: the test indenter is a quenched steel ball, and the hardness symbol is HBS;
< 650HB: The test indenter is cemented carbide with the hardness symbol HBW.
Empirical formula:
Mild steel: σb≈ 3.6HBS;
High carbon steel: σb≈ 3.4HBS.
Scope of application: For measuring gray cast iron, structural steel, non-ferrous metals and non-metallic materials.
Advantages and disadvantages:
- The measured value is accurate and the repeatability is good;
- Materials with heterogeneous tissue can be measured;
- Not suitable for testing finished products and thin parts;
- Measurement is time-consuming and inefficient.
(2) Rockwell hardness
The hardness value of the material is expressed by measuring the indentation depth, and every 0.002 mm is equivalent to 1 Rockwell hardness unit.
There are two types of indenters:
1. Diamond cone with cone angle α = 120 °,
2. Small quenched steel ball with diameter of Φ1.588 mm.
Rockwell hardness calculation formula:
HR=(k-h)/ 0.002
Pressure head 1: K = 0.2mm; Head 2: K = 0.26 mm.
| Ruler | Hardness symbol | Type of indenter | Total test force F/N | Measure the hardness range | Application example |
| C | HRC | Diamond cone | 1471 | 20-70 | Quenched steel, high hardness cast iron, pearlitic malleable cast iron |
| B | HRB | Φ1.588 mm steel ball | 980.7 | 20-100 | Mild steel, copper alloy, ferritic malleable iron |
| A | HRA | Diamond cone | 588.4 | 20-88 | Cemented carbide, hardened steel sheet, thin surface hardened steel |
Advantages and disadvantages:
- The test is simple, convenient and rapid;
- The indentation is small, and the finished product and the thin piece can be measured;
- If the data is not accurate enough, the average value of the three points shall be measured;
- Materials with uneven structure, such as cast iron, shall not be tested.
(3) Vickers hardness
The hardness value is calculated based on the test force per unit area of the indentation.
The indenter is a diamond quadrangular pyramid with an included angle of 136 degrees between two opposite surfaces.
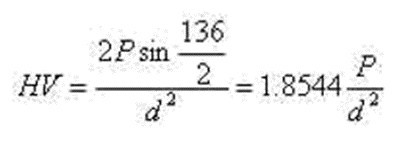
Measuring range:
It is often used to measure the thickness of parts, coatings, surface layers after chemical heat treatment, etc.
Advantages and disadvantages:
- Accurate measurement, wide range of applications (hardness from very soft to very hard);
- Measurable finished products and thin parts
- The surface of the sample is required to be high and labor-consuming.
Impact toughness
The ability of a material to resist failure under impact loading.
The impact energy Akconsumed when the sample is broken is:
Ak = m g H – m g h (J)
The impact toughness value akis the impact energy consumed per unit cross-sectional area at the notch of the specimen.
a k = Ak / S0 (J/cm²)
Low akvalues-Brittle materials:
There is no obvious deformation at the time of fracture, and the metal luster is crystalline.
High akvalue-tough material:
The plastic deformation is obvious, and the fracture is gray, fibrous and lusterless.
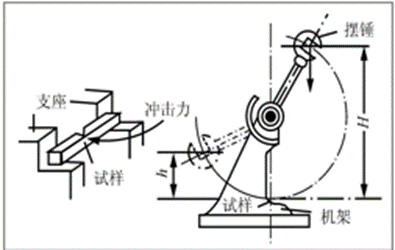
Fracture toughness
Fracture mechanics: On the premise of recognizing the existence of macroscopic cracks in machine parts, various new mechanical parameters for crack propagation are established, and the fracture criterion and material fracture toughness of cracked bodies are proposed.

Fatigue
Fatigue:
The fracture phenomenon of metal parts or components caused by cumulative damage under the long-term action of changing stress and strain.
Fatigue characteristics:
(1) Fatigue is a low-stress cyclic delayed fracture, and the fracture stress is often lower than the tensile strength of the material, or even the yield strength;
(2) Fatigue is a brittle sudden fracture, and there is no obvious sign of deformation before fracture, so it is dangerous;
(3) Fatigue is very sensitive to notches, cracks and structural defects, and is highly selective.
Fatigue limit σ-1:
The highest value of stress at which a material undergoes numerous stress cycles without fatigue fracture.
Conditional Fatigue Limit:
Maximum value of stress to be subjected to 107stress cycles without fracture.
Empirical formula for fatigue strength of steel:
σ-1 = (0.45~0.55)σb
Or σ-1= 0.27 (σs+ σb)
σ-1p = 0.23(σs+σb)
02 Heat treatment process
Definition: The process of changing the internal structure of solid metal or alloy through heating, heat preservation and cooling to obtain the required properties.
Purpose: First, to improve the material processing performance, to ensure the smooth follow-up processing, this heat treatment is called pre-heat treatment; The second is to improve the performance of materials and prolong the service life of parts, which is called final heat treatment.
Heat treatment classification:
Ordinary heat treatment (four heats: annealing, normalizing, quenching and tempering)
Surface heat treatment (surface hardening, chemical heat treatment)
Other heat treatments (vacuum heat treatment, thermomechanical treatment, etc.)
Microstructure transformation of eutectoid steel during heating
There are four steps in the transformation process from pearlite to austenite:
(1) Austenitic nucleation;
(2) growth of austenite;
(3) dissolve that rest Fe3C;
(4) Austenite homogenization.
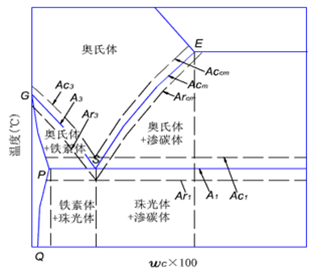
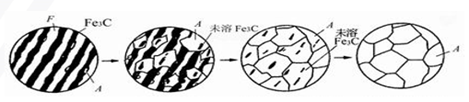
Structural transformation of steel during cooling
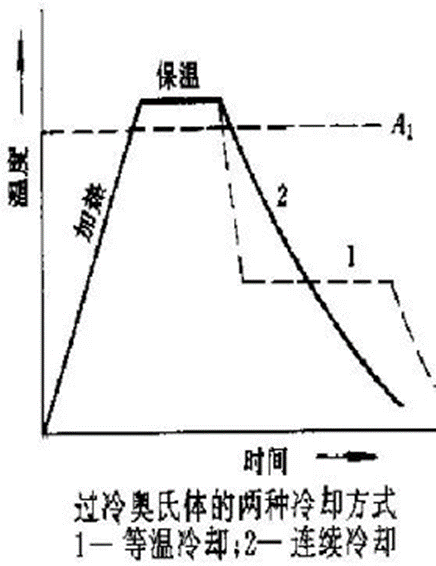
Cooling transformation of austenite: austenite is a stable phase above the critical point A1, and becomes an unstable phase when cooled below A1, and structural transformation will occur.
Importance: Determines the structure and properties of steel after heat treatment. For the same kind of steel, the heating temperature and holding time are the same, but the cooling method is different, and the properties after heat treatment are quite different.
Mechanical properties of 45 steel after heating to 840 ℃ and cooling under different cooling condition
| Cooling method | σb/Mpa | σs/Mpa | δ/% | ψ/% | HRC |
| Cooling with the furnace | 519 | 272 | 32.5 | 49 | 15~18 |
| Air Cooling | 657~706 | 333 | 15~18 | 45~50 | 18~24 |
| Cooling in oil | 882 | 608 | 18~20 | 48 | 40~50 |
| Cooling in water | 1078 | 706 | 7~8 | 12~14 | 52~60 |
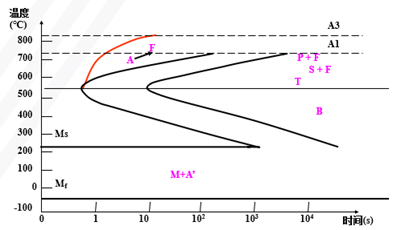
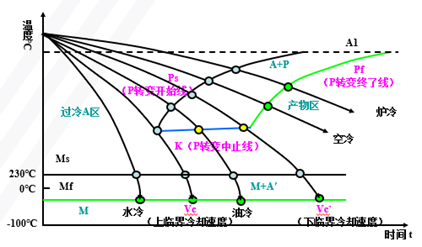
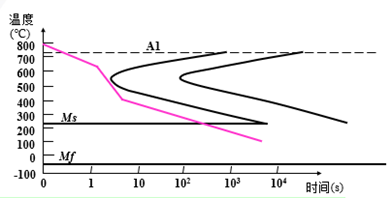
Establishment of Isothermal Transformation Curve of Undercooled Austenite in Eutectoid Steels (Metallographic Hardness Method)
Also known as “TTT Curve” (Time-Temperature-Transformation Curve), because the shape is similar to “C”, it is often called “C Curve”.
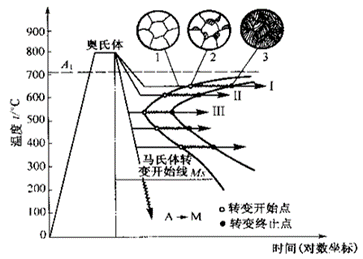
With the help of “C curve”, we can understand what kind of structure the austenite transforms into under different cooling conditions and the properties of the transformed products, which provides a theoretical basis for the correct formulation and selection of heat treatment process.
C-curve and transformation product of eutectoid steel
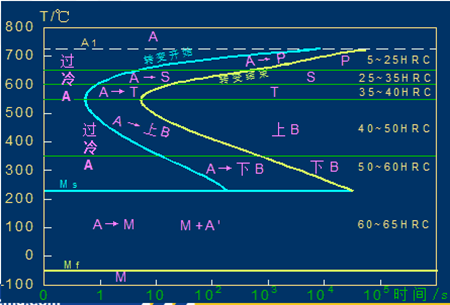
1) Pearlitic transformation (also known as high-temperature transformation)
Transition temperature: A1-550 ℃; Transformation product: pearlite
A1~ 6500C: coarse lamella of pearlite, P (pearlite-pearlite)
6500C ~ 6000C: fine pearlite lamellae, S (sorbite)
6000C ~ 5500C: very fine lamellae of pearlite, T (troolstite)

The lamellar size of ferrite and cementite in pearlite is related to the transformation temperature. The lower the temperature, the finer the lamellae of pearlite. The lamellae become thinner, the strength and hardness increase, and the plasticity and toughness increase.
2) Bainitic transformation (also called medium temperature transformation)
Transition temperature: 550 — Ms (230 ℃)
Transformation product: bainite — a mixture of supersaturated F and cementite.
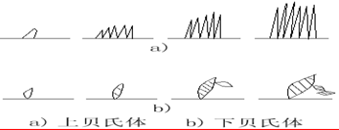
550 ~ 350 ℃: upper bainite (BGo) feathery structure with low strength and plasticity and high brittleness.
350 ℃ ~ Ms: lower bainite (BDown) with needle-like structure and good comprehensive properties.
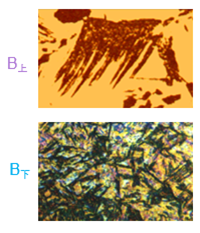
3) Martensitic transformation (also called low temperature transformation)
Transition temperature: Ms (230°C) ~ Mf
Transformation product: martensite + A ‘ (residual austenite)
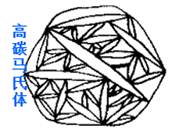
Martensite: a supersaturated solid solution of carbon in α-Fe, denoted by M.
Classification
Low-carbon martensite (low carbon martensite): in the shape of lath, with high strength and ductility. Also called lath martensite.
High-carbon martensite (high carbon martensite): It takes the shape of a lens, with a ridge line in the middle. Its strength is very high, but its plasticity and toughness are poor, and its brittleness is high.

C-curve of hypoeutectoid steel
C-curve of hypereutectoid steel
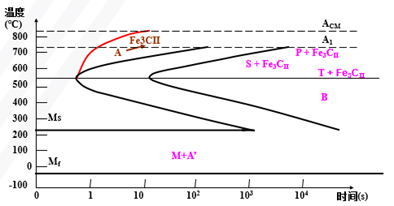
Continuous Cooling Transformation (CCT) Curve of Supercooled Austenite
Annealing
Definition: Metal is heated to a certain temperature, held for a sufficient time, and then cooled at a suitable rate
Purpose
- Refining grains;
- Reduce the hardness and improve the formability and machinability of steel
- Eliminate internal stress.
Classification: According to the purpose and process characteristics of annealing, it can be divided into complete annealing, incomplete annealing, isothermal annealing, spheroidizing annealing and stress relief annealing.
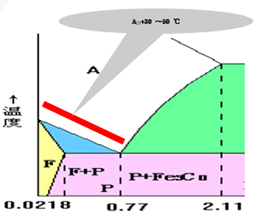
Full annealing
Scope of application: hypoeutectoid steel
Heating temperature: Ac3+ 30 ~ 50 ℃
Objective: To refine the structure, reduce the hardness and improve the machinability.
Eliminate internal stress
Room temperature structure: F + P
Spheroidizing annealing
- Applicable scope: eutectoid steel and hypereutectoid steel
- Heating temperature: Ac1+ 20 ~ 30 ℃
- Objective: To spheroidize Fe3CⅡin the form of net or flake.
- Structure: spherical pearlite
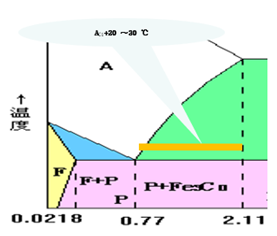
Isothermal annealing
- Process: heat to Ac1+ 30 ~ 50 ℃ or Ac3+ 30 ~ 50 ℃, after heat preservation, quickly cool to a certain temperature below Ar1, when A becomes P type tissue, take out of the furnace and cool in the air.
- Organization: Class P
- Has the advantages of short annealing time and uniform structure.
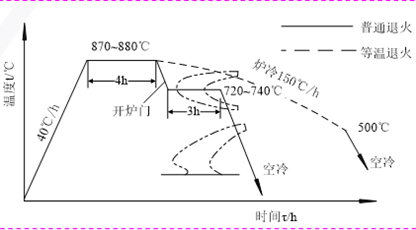
Stress relief annealing
- Purpose: to remove residual stress
- Heating temperature: THeating< AC1(500 ~ 600 ℃)
- Application: Eliminate residual internal stress in castings, forgings, weldments, etc.
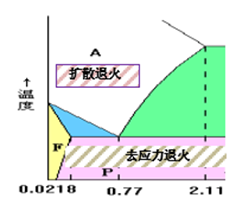
Homogenization annealing (diffusion annealing)
- Objective: To eliminate segregation; Homogeneous composition and structure
- Heating temperature: AC3+ 150 ~ 250 ℃
- Structure: hypoeutectoid steel is P + F.
- Application: Mainly used for alloy steel ingots, castings and forgings with high quality requirements.
Recrystallization annealing (recrystal lization annealing)
- Process: heating to 50-150 ℃ below Ac1, or TAgain+ 30-50 ℃, heat preservation and slow cooling.
- Purpose: To eliminate work hardening and restore the plasticity and toughness of steel.
- Application: Eliminates work hardening of workpieces after cold working. For example, in the process of drawing steel wire, annealing is carried out in the middle.
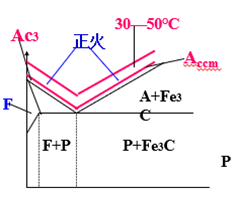
Normalizing
Definition: a heat treatment process in which the workpiece is heated to 30 ~ 50 ℃ above Ac3or Accm, and then taken out of the furnace for cooling in the air after heat preservation.
Purpose
Low carbon steel: improve hardness, easy to cut.
Hypereutectoid steel: eliminate secondary cementite network, which is beneficial to P spheroidization.
Medium carbon steel and medium carbon low alloy steel: they can be used as final heat treatment due to low stress and low performance requirements.
Quench
Objective: To obtain M or BDownstructure and improve the hardness and wear resistance of the steel.
Selection of quenching temperature
- Hypoeutectoid steel: AC3+ 30 ~ 50 ℃;
- Eutectoid steel and hypereutectoid steel: AC1+ 30-50 ℃.
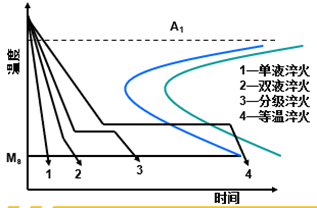
Quench cooling is the key to determine the quality of quenching, and the ideal cooling rate should be as shown in the figure.
Above 650 ℃, slow, reduce thermal stress
650-400 ℃, fast, avoid C curve
Below 400 ℃, slow, reduce transformation stress
Common quenching medium
At present, oil, water and saline are commonly used as cooling media in production, and their cooling capacity increases in turn.
Water: The quenching ability is strong, but there are soft spots on the surface of the workpiece, which is easy to deform and crack.
Saline: the quenching ability is stronger, the surface of the workpiece is smooth, without soft spots, but it is easier to deform and crack;
Oil: the quenching ability is weak, but the workpiece is not easy to deform and crack
Common quench cooling method
Tempering
Definition:
The main purpose of tempering
- Eliminate internal stress and reduce brittleness
- Stable organization and workpiece size
- Reduce hardness and improve plasticity
Structure and property changes of tempering
The microstructure transformation of quenched steel during tempering mainly occurs in the heating stage. With the increase of heating temperature, the microstructure of the quenched steel changes in four stages.
1. Decomposition of martensite
Tempering stage: when tempering at 100 ℃, there is no change in the structure; Martensite decomposes when heated at 100-200 ℃.
Structure obtained: tempered martensite MBack(supersaturated alpha solid solution).
Performance change: the internal stress is gradually reduced, and the performance is basically unchanged.
2. Decomposition of residual austenite
Tempering stage: 200-300 ℃. A ′ decomposes into BDown.
Acquired organization: MBack(Tempered Martensite) indicates
Changes in properties: stress is further reduced, and strength and hardness are slightly reduced.
3. Martensite decomposition completion and cementite formation
Tempering stage: 300-400 ℃. The epsilon carbide is transformed into stable cementite.
Structure obtained: Tempered troostite, represented by TBack(F + very fine granular Fe3C).
Performance change: the internal stress is basically eliminated, the hardness is reduced, and the plasticity and toughness are increased.
4. aggregation and growth of Fe3C and recovery and recrystallization of α solid solution
Tempering stage: above 400 ℃. The α phase begins to recover, and when the temperature is above 500 ℃,
Raw recrystallization;
The obtain structure: Tempered sorbite, represen by SBack(F + fine-grained Fe3C).
Performance change: good comprehensive performance is obtained.
Microstructure and mechanical properties of steel after tempering
| Craft | Tempering temperature (℃) | Tempered Structure | Hardness after tempering (HRC) | Performance characteristics | Use |
| Low temperature tempering | 150~250 | MBack | 58~64 | High hardness, high wear resistance; Brittleness and reduction of internal stress | Tool steel, Rolling bearing, carburized parts, etc. |
| Medium temperature tempering | 250~500 | TBack | 35~50 | Higher elastic limit and yield limit, with certain plasticity and toughness | Spring steel, Hot work mold |
| High temperature tempering | 500~600 | SBack | 25~35 | Ood comprehensive performance | Important structural parts |
The general trend of the change of mechanical properties during tempering is that with the increase of tempering temperature, the strength and hardness of steel decrease, while the plasticity and toughness increase.
Surface Heat Treatment
Surface heat treatment: a heat treatment process in which only the surface layer of a workpiece is heat treated to change its structure and properties.
Classification: Surface hardening and chemical heat treatment.
In production, there are many parts that require the surface and core to have different properties, generally high surface hardness, high wear resistance and fatigue strength; The core is required to have better plasticity and toughness.
In this case, the material selection alone or the use of ordinary heat treatment methods can not meet its requirements. The way to solve this problem is surface heat treatment.
Urface quenching
Definition: a heat treatment process in which only the surface of the workpiece is quenched (+ tempered).
Purpose: to make the surface of the workpiece hard and the heart tough.
Steel for surface hardening: medium carbon structural steel (carbon content 0.4% -0.5%)
The method comprises induction heating surface quenching and flame heating surface quenching.
Induction surface hardening (induction surface quenching)
Basic principle: AC current is applied to the induction coil → eddy current is formed (skin effect) → A of the surface layer → M of water cooling.
Classification
High frequency induction heating:
200~300kHz,0.5~2.5mm;
Medium frequency induction heating:
0.5~10kHz,2~10mm;
Power frequency induction heating:
50Hz,10~20mm。
Law: that great the frequency of the current,
The shallower the depth of the hardened layer.
Flame hardening (flame heating surface quenching)
Definition: Flame heating surface quenching refers to quenching by heating the surface of parts with oxygen-acetylene (or other combustible gas) flame and then rapidly cooling. The depth of hardening layer is generally 2 ~ 6mm.
Application: Suitable for single piece and small batch production.
Chemical heat treatment of steel (chemical heat treatment)
Definition: a heat treatment process in which a steel part is placed in an active medium at a certain temperature for heat preservation, so that one or more elements penetrate into its surface to change its chemical composition, structure and properties.
Classification: According to different elements, chemical heat treatment can be divided into carburizing, nitriding, carbonitriding, boronizing, aluminizing and so on
Basic process:
(1) decomposition, namely decomposing the chemical medium into active atoms of infiltration elements in the process of heating and heat preservation;
(2) absorption: that active atom are adsorbed by the surface of the workpiece to form a solid solution or a special compound;
(3) diffusion: that infiltrate atoms diffuse inward from the surface lay of the workpiece to form a diffusion layer with a certain depth, namely an infiltration layer.
Carburization of steel (Carburize of steel)

Purpose: To improve the hardness and wear resistance of the workpiece surface
Steel for carburizing: low carbon steel or low carbon alloy steel, such as 20, 25
Medium: The most commonly used gas (kerosene, benzene, etc.), with active carbon atoms.
Temperature: 900-950 ℃ in austenitic region
Time: about 10 hours, depending on the depth of the infiltration layer.
Structure after carburization: if the workpiece is slowly cooled after carburization, the structure from the surface to the core is
P+Fe3CⅡ→P→P+F。
Other methods of chemical heat treatment
Nitriding: a heat treatment process in which active nitrogen atoms penetrate the surface of a workpiece at a certain temperature. And improve that surface hardness, wear resistance, fatigue strength, hot hardness, corrosion resistance and the like of the part.
Carbonitriding: Carbon and nitrogen simultaneously penetrate into the surface of the workpiece. Improves the surface hardness, the fatigue resistance and the wear resistance, and has the advantages of carburization and nitriding.
Chromizing: It has good corrosion resistance and excellent oxidation resistance, hardness and wear resistance, and can replace stainless steel and heat-resistant steel for tool manufacturing.
Boronizing: very excellent wear resistance, corrosion wear resistance and mud wear resistance, wear resistance is significantly better than nitriding, carbon and carbonitriding layer, but not resistant to atmospheric and water corrosion. It is mainly used for mud pump parts, hot work molds and workholding fixtures.

